Projects from Make: Magazine
Electrolysis Rust Remover
A homemade rust remover that's easy and uses common materials.

A homemade rust remover that's easy and uses common materials.


Nasty, rusty lathe tools.



Get a battery charger, plastic bucket washing soda (not soap!), some plain steel wire (no stainless, ever!) and a stick or plastic pipe. Next is the fun part, but it is not the safest.
THIS PROCCESS CREATES HYDROGEN AND OXYGEN GASES WHICH ARE VERY EXPLOSIVE! THE SOLUTION WILL REMOVE THE OILS FROM YOUR HANDS! Be careful, ventilate, no sparks, and wear gloves.


In your PLASTIC bucket pour some clean water and about 1 tablespoon per gallon of washing powder. The amount does not have to be precise.
Then with plain steel wire make a cage to closely fit the inside of your bucket, all electrically connected, with a lead above the edge.
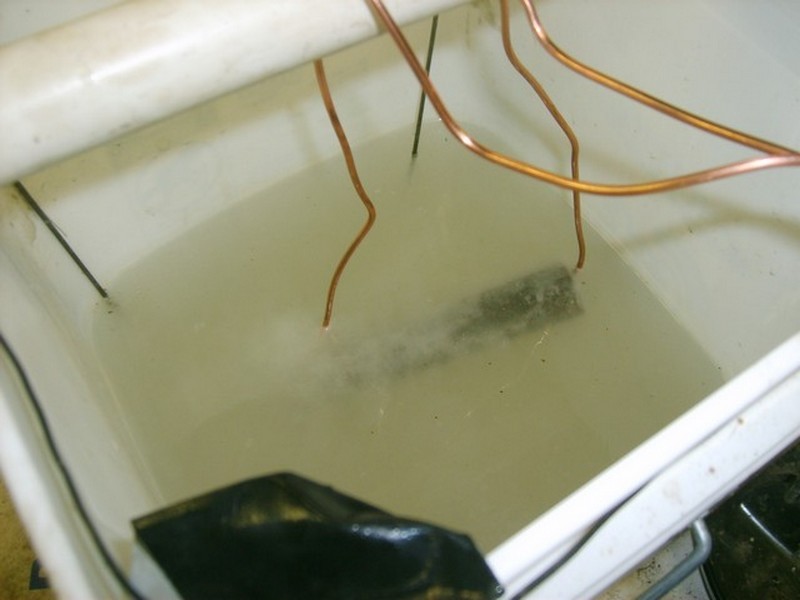
Suspend your rusty part from the pipe or stick with wire, submerged, making sure it does not touch the steel basket lining the bucket. Do not use copper like I did. Use steel wire, the same as for the cage. Make sure the two poles ( positive and negative ) never touch.
Make sure your work area is well vented! Unplug your 12-volt battery charger. Set it to about 2 amps. Hook the positive (red) lead to the bucket basket and the negative (black) lead to the rusty part lead. Plug the charger in and look at the meter; it should be drawing about 2 amps. If it is a lot more or things are smoking, UNPLUG! The part will slowly start fizzing. When it stops (a couple of hours later, depending on the part), unplug your charger and remove your part. Clean the black slime off of it and paint or oil it to protect it.
The water is NOT toxic; it is safe to pour down a drain. It is just soap and steel. The process should have removed any loose paint also.

Your parts should look like this now! All this and more at http://www.toolfools.com/forum!
This can be made from scrap materials and recycled or re-used when finished. It is very simple to make when you understand the simple process, You can improve on your next one! It will not last, the cage is a consumable. Build it custom for each project, do not over-think this. K.I.S.S.