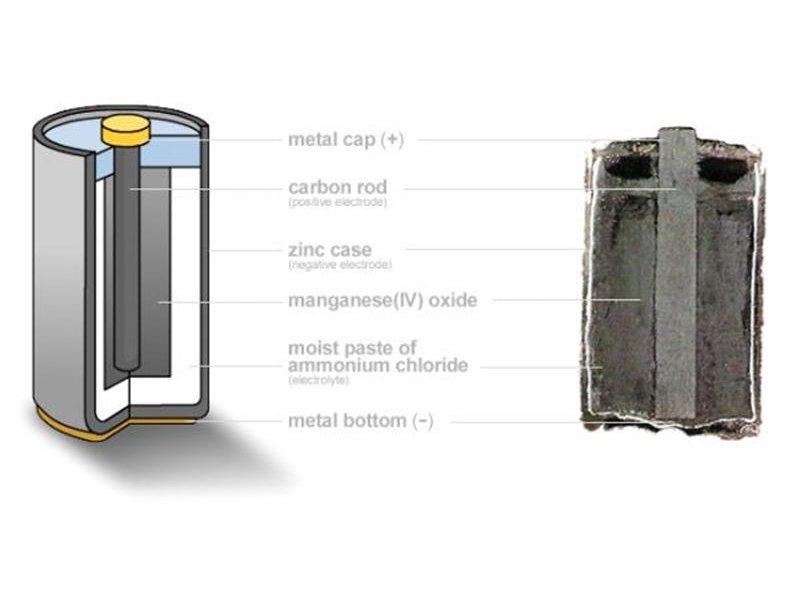
This tutorial shows how to take apart a spent zinc-carbon dry cell of the common household type. Besides making for an interesting object lesson in electrochemistry, taking apart a spent D-cell, for instance, allows you to salvage many materials which can be of use to amateur chemists–materials which would otherwise probably end up in a landfill. Separated from their reactive components, the leftover parts of the battery can be safely added to most municipal recycling streams.
A zinc carbon cell (Wikipedia) contains manganese dioxide, which, among other things, is useful as a catalyst in the production of oxygen gas from hydrogen peroxide. It also contains metallic zinc, which can be used, for instance, as a reagent in the production of hydrogen gas from strong acid. Finally, it contains a carbon or graphite rod which can be used as an electrode in any of a number of electrochemical experiments, such as the electrolysis of water and the construction of an arc light or arc furnace.
Note that the battery in this tutorial is a zinc-carbon dry cell. This tutorial does not cover the dismantling of an alkaline-type cell. Alkaline cells are of slightly different internal construction and contain the strong base potassium hydroxide as an electrolyte, which is rather more dangerous to handle than the ammonium chloride/zinc chloride mixture used in zinc carbon cells. Zinc-carbon cells are commonly labelled “general purpose” or “heavy duty,” and will not have the word “alkaline” on the case.