Each month this year, we’re exploring a different electronic component, delving into what it is, how it works, and how you use it in projects. Last month we looked at the good ol’ switch. This month, it’s the battery, that portable power house that provides the electronic nutrition circuits need to come to life. As always, we’ll start things off with an introduction to batteries via an edited excerpt from Charles Platt’s essential Encyclopedia of Electronic Components Volume 1: -Gareth Branwyn
 What goes on, exactly, inside those AAA batteries powering your LED flashlight? The short answer is: Chemistry. Chemical reactions can encourage electrons to flow out from one terminal and back to the other, doing some useful work along the way. Meanwhile, inside the battery, positive ions, also known as electron-holes, are changing places. Eventually the chemical reactions run down, and the battery stops delivering power. If it is a rechargeable type, you can force the electrons and the positive ions to go back where they started, ready to run again.
What goes on, exactly, inside those AAA batteries powering your LED flashlight? The short answer is: Chemistry. Chemical reactions can encourage electrons to flow out from one terminal and back to the other, doing some useful work along the way. Meanwhile, inside the battery, positive ions, also known as electron-holes, are changing places. Eventually the chemical reactions run down, and the battery stops delivering power. If it is a rechargeable type, you can force the electrons and the positive ions to go back where they started, ready to run again.

Figure 1
Figure 2
Figure 1 shows a cross-section of an everyday alkaline battery, and Figure 2 shows some schematic symbols. The ones in the top row are functionally identical with the ones in the bottom row.
The longer of the two lines in a battery symbol represents the positive side. One way to remember this is by imagining that the longer line can be snipped in half, so that the two segments can combine to form a + sign. Traditionally, multiple connected battery symbols indicate multiple cells inside a battery; thus the center symbols in Figure 2 could indicate a 3V battery, while those on the right would indicate a voltage greater than 3V. In practice, this convention is not followed conscientiously.
Batteries vs. Capacitors
Figure 3
Why can’t we just use big capacitors instead of batteries? A capacitor doesn’t require temperamental chemical rections, and theoretically can be recharged an unlimited number of times. In fact there are supercapacitors which have some specialized applications, but they cost a lot, they don’t hold their charge over long periods, and they store less electricity than a battery of the same weight. As shown in Figure 3, a capacitor also loses voltage much more rapidly during the discharge cycle. For the foreseeable future, we’ll be using batteries for portable power.
Disposables
In a world full of sophisticated rechargeable batteries, why do we still use disposables? First, their energy density is higher, and second, they have a shelf life of 5 years or more, because they lose their charge so slowly (this is known as the “self-discharge rate”). For applications such as smoke detectors, handheld remotes, or emergency flashlights, disposable batteries have no substitute. They have limits, though: They can’t deliver as much current as quickly as rechargeables.
Rechargeables
The most common types are “lead-acid,” “nickel cadmium” (abbreviated “nicad” or “NiCd”), “nickel metal hydride” (abbreviated “NiMH”), “lithium-ion” (abbreviated “Li-ion”), and “lithium-ion polymer.”
Lead-acid batteries have existed for more than a century. They contain lead plates which may be formed into a sponge texture, to maximize reactive surface area, although this texture can be physically abraded by deep discharge. In a deep cycle battery, the plates are solid. They are better able to withstand a discharge almost down to zero, but are less able to supply high amperage.
Figure 4
A sealed lead-acid battery intended to power an external light activated by a motion detector is shown in Figure 4. This unit weighs several pounds and is trickle-charged during the daytime by a 6″ x 6″ solar panel.
Figure 5
Nickel-cadmium (“NiCad”) batteries can withstand extremely high currents, but have been banned in Europe because of the toxicity of metallic cadmium. They are being replaced by nickel-metal hydride (“NiMH”) types, which are free from the “memory effect” that can prevent a NiCad cell from fully recharging if it has been left for weeks or months in a partially discharged state. Figure 5 shows a ten-pack of NiMH cells, each cell being the size of an alkaline D-cell. A pack like this is just the thing to drive a fair-sized robot.
Figure 6
Some small rechargeable batteries are shown in Figure 6. The NiCad pack at top left was manufactured for a cordless phone, and is rapidly becoming obsolete. The 3V lithium battery at top right was intended for a digital camera. The three batteries in the lower half of the photograph are all rechargeable NiMH substitutes for 9V, AA, and AAA batteries. The NiMH chemistry results in the AA and AAA single-cell batteries being rated for 1.2V rather than 1.5V, but the manufacturer claims they can be substituted for 1.5V alkaline cells because NiMH units sustain their rated voltage more consistently over time. Thus, the output from a fresh NiMH battery may be comparable to that of an alkaline battery that is part-way through its discharge cycle.
Amperage
Because ion transfer must occur inside the battery to complete the circuit, the current that a battery can deliver will be limited by its internal resistance. Any type of rechargeable battery has a lower internal resistance than alkalines.
Figure 7
Since a battery will deliver no current if there is no load, current must be measured while a load is attached, and cannot be measured by a meter alone. The fuse in the meter will blow if the meter is connected directly between the terminals of a battery. Current must always be measured with the meter in series with a load. See Figure 7.
Capacity
Figure 8
The electrical capacity of a battery is measured in amp-hours, abbreviated “Ah,” “AH,” or (rarely) “A/H.” Smaller values are measured in milliamp-hours, usually abbreviated “mAh.” If I is the current being drawn from a battery (in amps) and T is the time for which the battery can deliver that current (in hours), the amp-hour capacity is found by multiplying I by T. In reality, there are major limits to this formula, because the chemistry of batteries limits their ability to delivery high currents. Figure 8 shows some numbers claimed by a battery manufacturer, for low currents. In reality, even these numbers are optimistic, and the final voltage in each case may be unacceptably low for electronics applications.
Voltage
The rated voltage of a fully charged battery is known as the open circuit voltage, abbreviated OCV or VOC, defined as the potential that exists when no load is imposed between the terminals. Because the internal resistance of a volt meter (or a multimeter, when it is used to measure DC volts) is very high, it can be connected directly between the battery terminals with no other load present, and will show the OCV quite accurately, without risk of damage to the meter. A fully charged 12-volt car battery may have an OCV of about 12.6 volts, while a fresh 9-volt alkaline battery typically has an OCV of about 9.5 volts. Be extremely careful to set a multimeter to measure DC volts before connecting it across the battery. Usually this entails plugging the wire from the red probe into a socket separately reserved for measuring voltage, not amperage.
The voltage delivered by a battery will be pulled down significantly when a load is applied to it, and will decrease further as time passes during a discharge cycle. For these reasons, a voltage regulator is required when a battery powers components such as digital integrated circuit chips, which do not tolerate a wide variation in voltage.
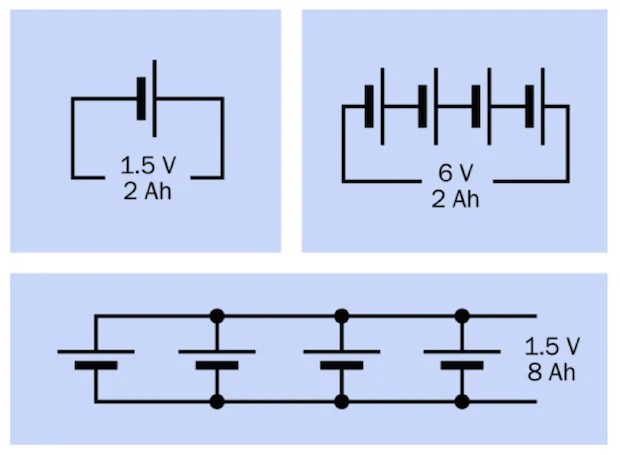
Figure 9
Batteries or cells may be used in series or in parallel. In series, the total voltage of the chain of cells is found by summing their individual voltages, while their amp-hour rating remains the same as for a single cell, assuming that all the cells are identical. Wired in parallel, the total voltage of the cells remains the same as for a single cell, while the combined amp-hour value is found by summing their individual amp-hour ratings, assuming that all the batteries are identical. See Figure 9.
What Not to Do
A battery capable of delivering significant current can overheat, catch fire, or even explode if it is short-circuited. Dropping a wrench across the terminals of a car battery will result in a bright flash, a loud noise, and some molten metal. Even a 1.5-volt alkaline AA battery can become too hot to touch if its terminals are shorted together. (Never try this with a rechargeable battery, which has a much lower internal resistance, allowing much higher flow of current). Lithium-ion batteries are particularly dangerous, and almost always are packaged with a current-limiting component which should not be disabled. A short-circuited lithium battery can explode.
If a battery pack is used as a cheap and simple workbench DC power supply, a fuse or circuit breaker should be included. Any device that uses significant battery power should be fused.

For more on microswitches, rockers, sliders, toggles, DIPs, SIPs, paddle switches, and more, check out the Encyclopedia of Electronic Components Volume 1 by Charles Platt. It’s the informative, concise, and well-organized resource that's perfect for teachers, hobbyists, engineers, and students wanting a go-to electronics quick reference.
ADVERTISEMENT