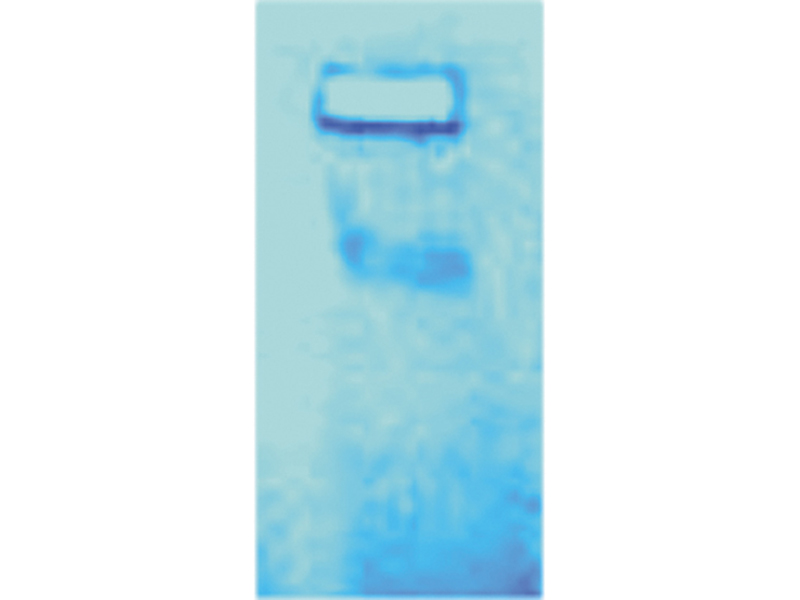
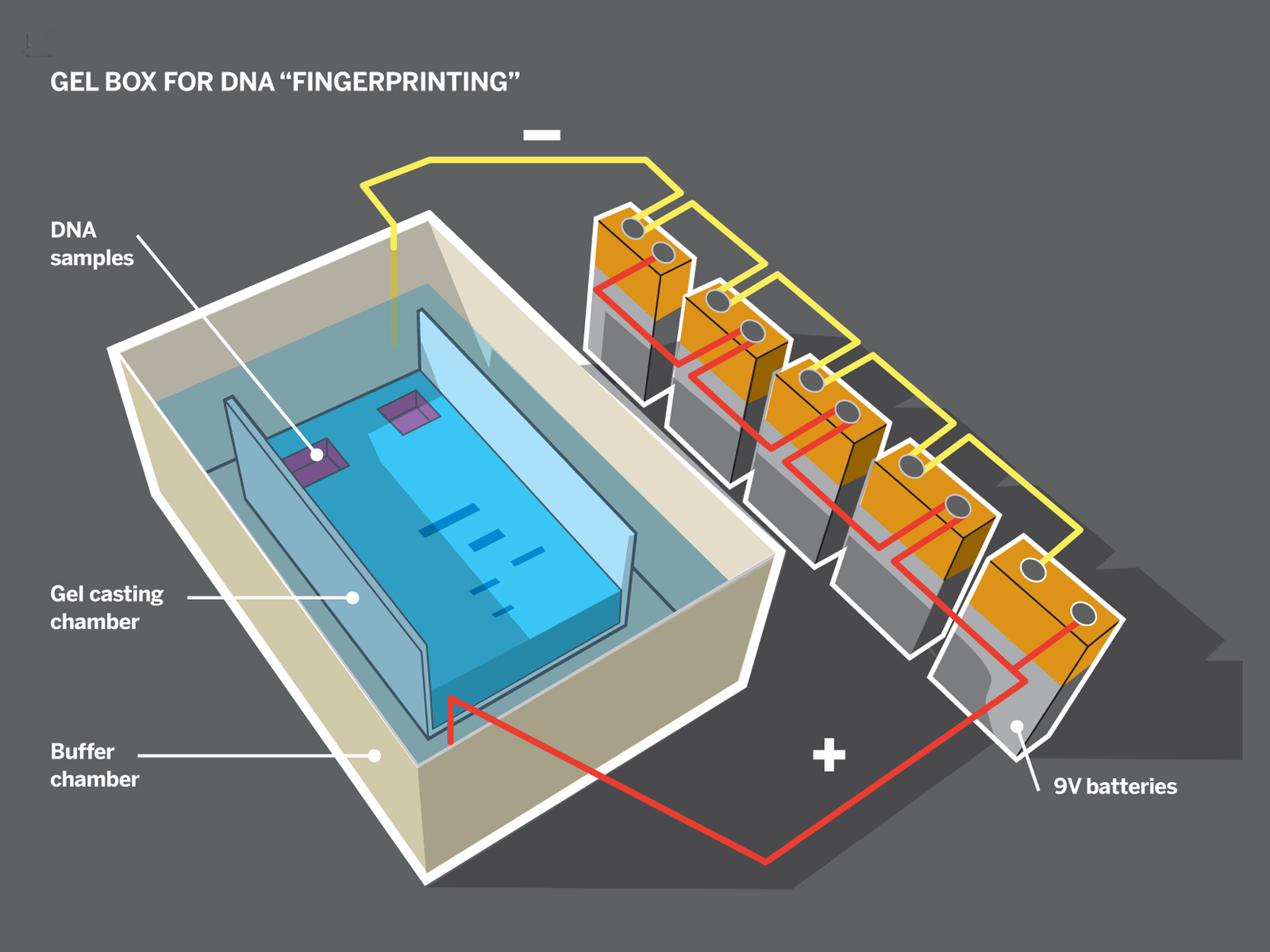
The gel electrophoresis process lets you separate and visualize different DNA molecules based on their sizes. Agar gel — a dense, microscopic network of sugar — is difficult for larger DNA molecules to travel through, but because DNA is negatively charged, you can draw it through the gel by applying an electric field. A gel box uses this principle to act as a DNA racetrack. The shorter the strand of DNA is, the faster it runs.
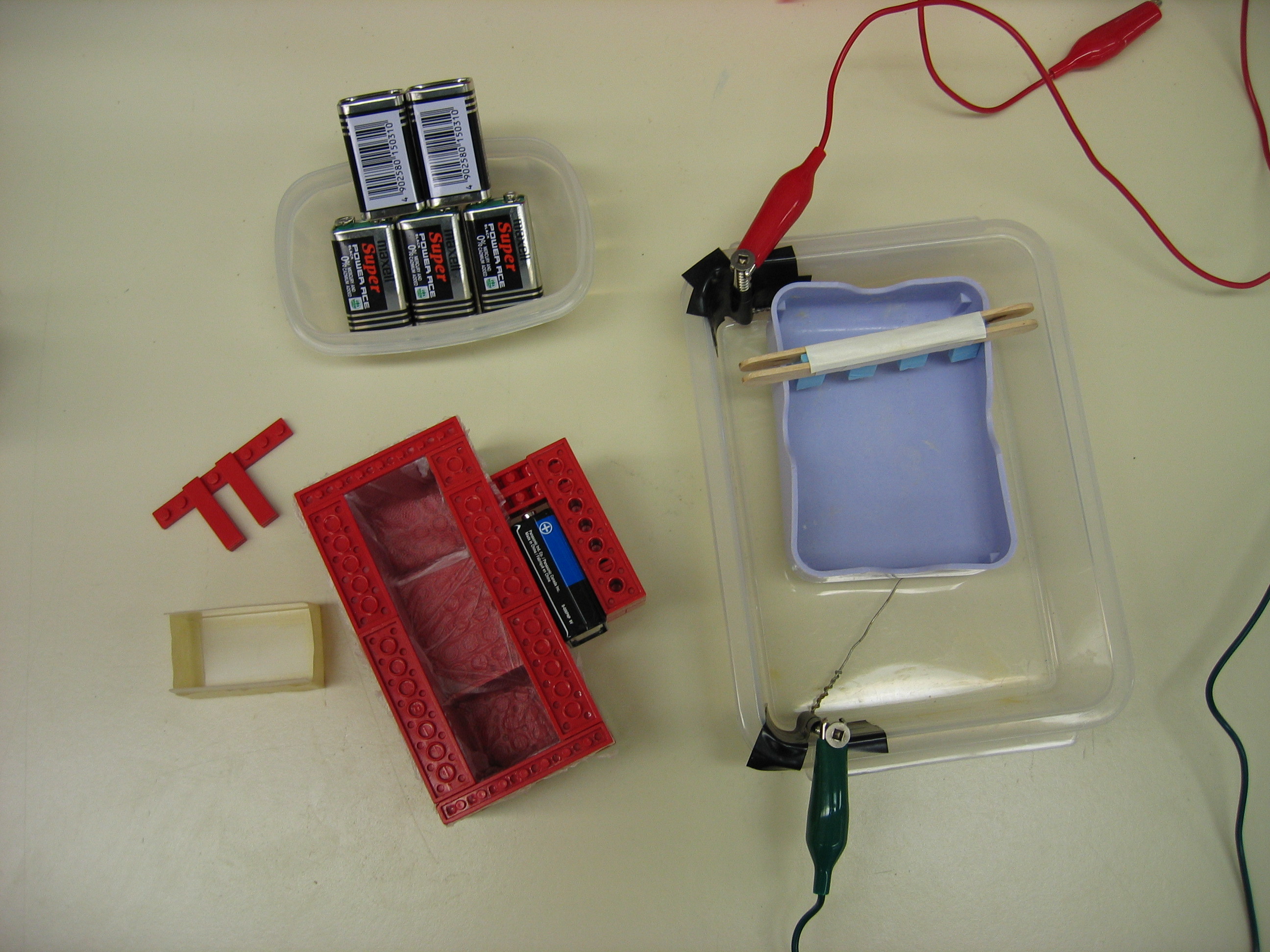
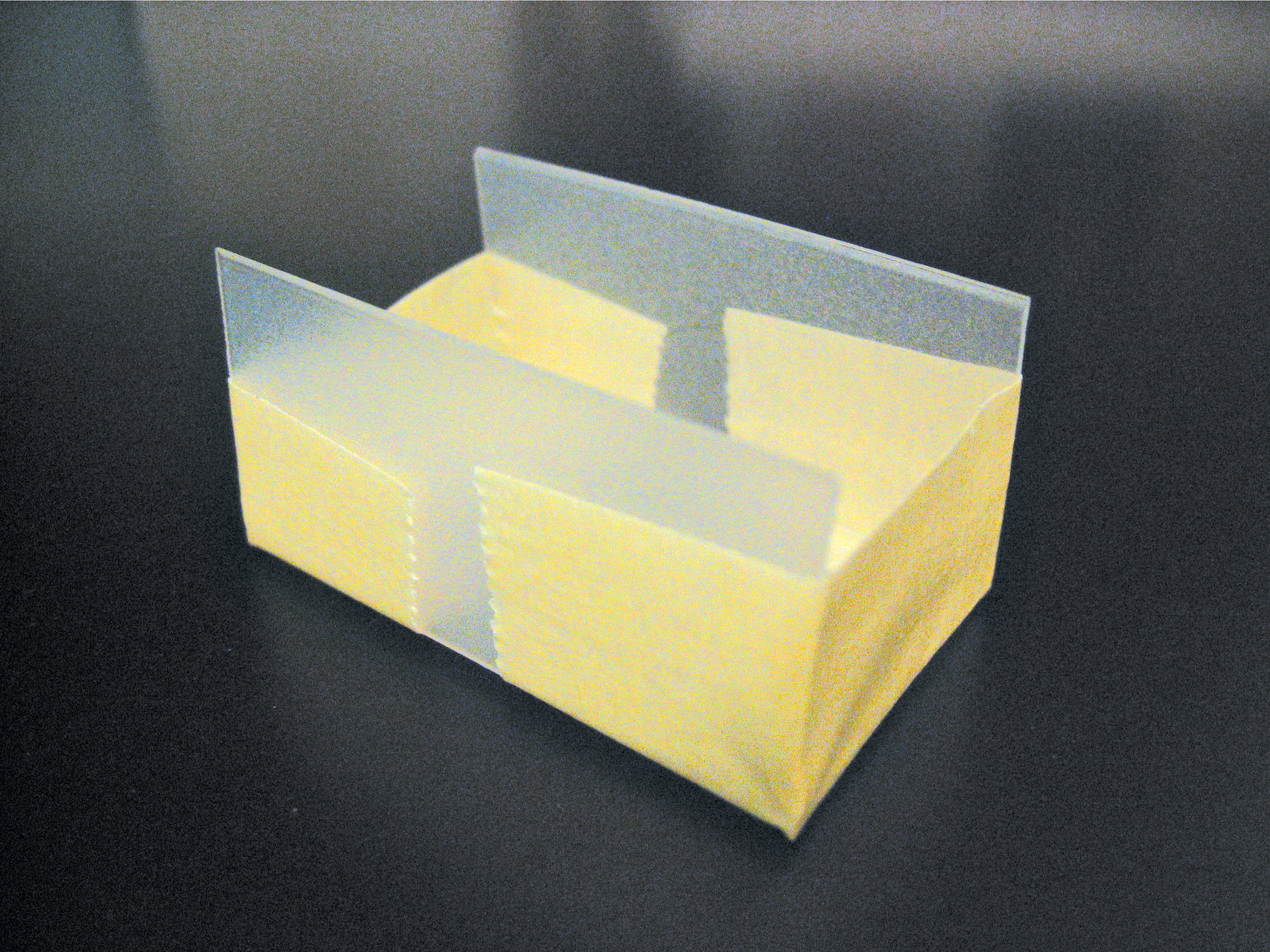
The box consists of 2 nested containers. An inner “casting chamber” contains the gel itself and sits in the running buffer inside the outer “buffer chamber.” Two opposite ends of the casting chamber are open to give the gel direct contact with the electrolytic buffer. Two corresponding electrodes in the buffer chamber electrify the liquid across the 2 ends.
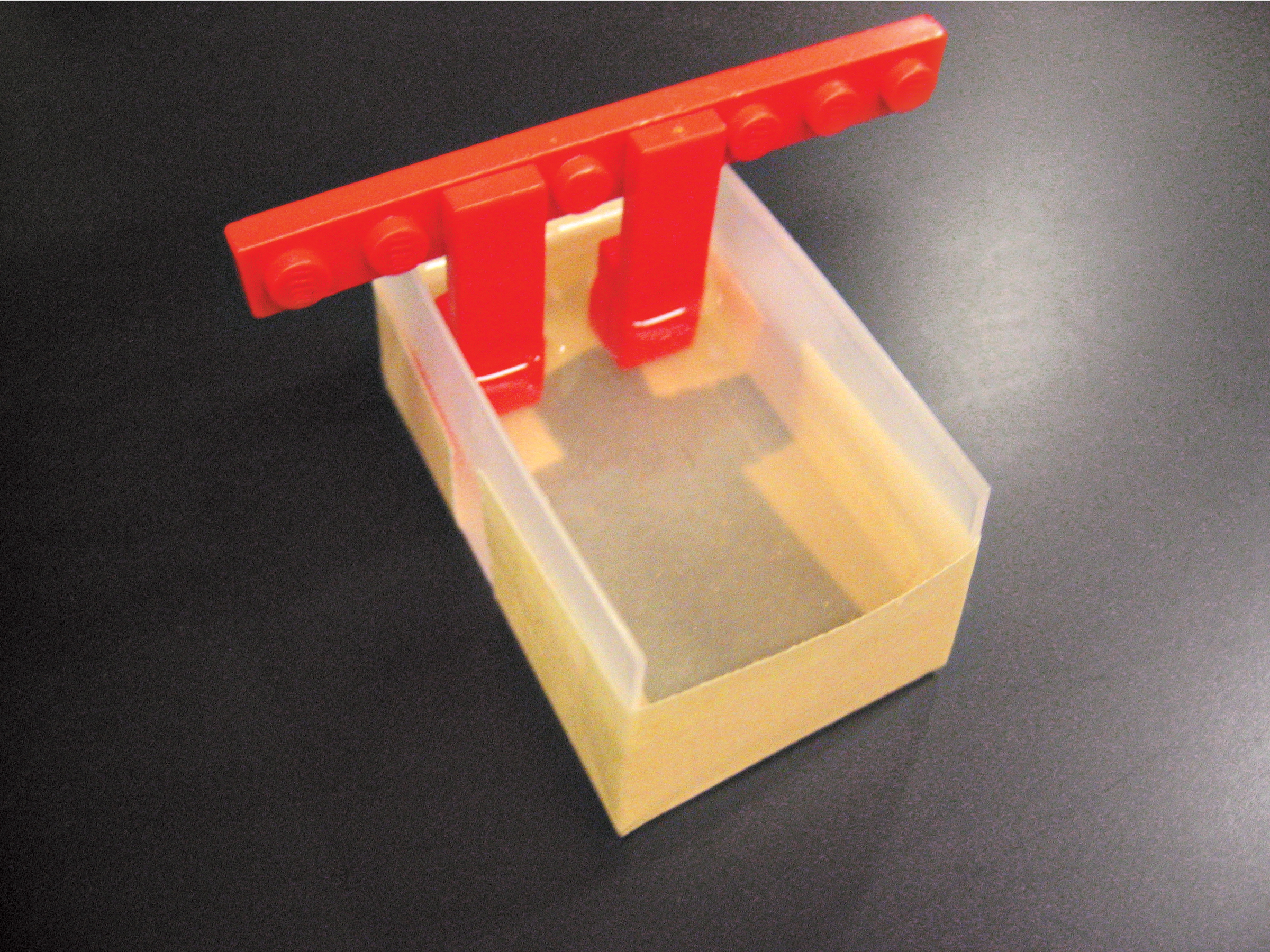
We built our box in 2 ways: using half of a plastic travel soap dish inside of a larger rectangular Tupperware container, and using the lid of a plastic slide box inside a box made of Lego blocks and lined with Glad Press ’n Seal wrap to prevent leakage. In both cases, we cut away the narrow sides of the casting chamber, creating a U-shaped channel. For the electrodes, we ran wire along both ends of the buffer chamber and connected each to a screw as a contact.