
From the MAKE Flickr photo pool
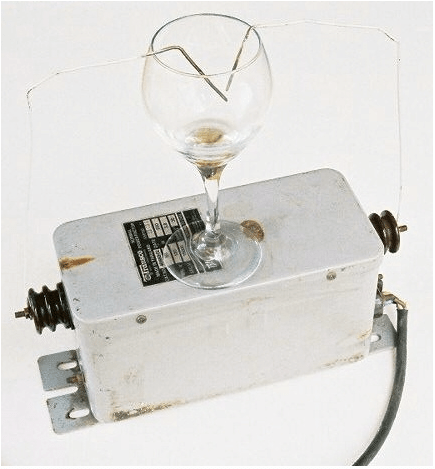
Eric “ALH84001” Archer conducted some testing on the ability of a neon sign transformer to improve beverage enjoyment –
Round 1: Lone Star (the national beer of Texas). 1 cm arc discharge to surface of cold beer for about 15 seconds. Slight foaming occured at the point of arc contact. Panelist 1 (EA) noted the acrid flavor of ozone in the beverage and tossed it over the fence.
Round 2: inexpensive red wine. both electrodes beneath liquid surface, about 10 seconds. transformer hummed but no visible arcing. Panelist 2 (DF) noted a decrease in the “bite” of the wine.
For more results see here – Apparatus for High Voltage Treatment of Alcoholic Beverage
14 thoughts on “Apparatus for High Voltage Treatment of Alcoholic Beverage”
Comments are closed.
ADVERTISEMENT
Join Make: Community Today











I’ve heard of this way of “aging” wine before. Passing current through an electrolyte leaves metal ions from the electrodes in the electrolyte. Metal ions are toxic. So have fun posioning yourself.
http://www.physorg.com/news145255770.html
Is being able to turn tequila into diamonds, through SCIENCE!
The Oracle: I don’t think the small amount of metal ions that may be produced is going to do nearly as much harm as you imply. We’re not talking about doing it for hours, not even minutes but seconds.
If I recall my electrochem, the quantity of metal ions will be proportional to the coulombs of charge that flow between the electrodes. Depending on how much current you get with that transformer even a few seconds could produce undesirable amounts of metal ions.
Of course, in this case the current is probably fairly low, so ehrichweiss’s conclusion is likely still correct. My point is that it’s coulombs that count, not seconds.
Also I’m not sure what the impact of the AC current will be. To a certain degree, metal ions formed around an electrode will be reabsorbed when the polarity changes. It’s not likely to prevent net metal ion generation, but it might reduce the total quantity.
If I recall correctly, a lot of nasty stuff can be produced by electrolysis, almost always poisonous or dangerous to drink. The author would better contact a chemist and describe exactly the process before drinking anything, unless he’s candidating himself for a Darwin Award.
@Perry and Josh — the great thing about this hobby is that a little bit of knowledge is a dangerous thing, someone like ehrichweiss is so sure he’s right he’ll take himself out of the gene pool and make humanity just a bit smarter.
—a little bit of metal ions aren’t dangerous — tell that to the people who drake out of lead containers or used mercury to make hats, or a thousand other examples. There’s links between alzehiemers and aluminum, but until very recently, aluminum cookware was very common.
But erichweiss *thinks* the metal ion concentration won’t be significant. Hey here’s a little bit of info for you. A lot metal ions (mercury, silver, and aluminum for example) *never* leave your body, it’s lifetime exposure that counts and in that context, every single ion is one too many.
Me thinks you might try to make a flaming Homer from the Simpson’s. It might be a jumping off point.