I received this note from Cory Tobin several months ago. It was part of an email chain started by our mutual friend, Mac Cowell. Mac told me that Cory was working on an an interesting biology project that began in his apartment, and was now flourishing at the LA Biohackers space. I had to know more.
I referenced Cory’s project in a previous post about the different types of amateurs who are redefining and reimagining citizen science and exploration.
Here’s Cory:
September 9
Cory Tobin
9/9, 1:54amOK, I don’t want to make any assumptions about your bio/chem knowledge so I’ll just give you my typical spiel starting from the basics. Don’t be offended if you already know this stuff. Anyways, here it is…
All living stuff needs nitrogen to build proteins. Nitrogen is quite abundant in the atmosphere (78%) but it’s in the form of N2 which is a very stable molecule, so using it directly is near impossible. Humans get some nitrogen from plant proteins or more so from meat which, while the meat was still alive, got it from eating plants. Most plants get nitrogen from the soil either in the form of ammonium, nitrates, free amino acids or to a lesser degree, nitrites. These compounds are mostly the result of other decaying organisms. But where did it come from originally? At some point someone had to get it from the atmosphere, otherwise there would be a net loss as these nitrogen-containing compounds are washed into the ocean or converted into nitrogen gas and return to the atmosphere.
Before humans started interfering the only terrestrial source of nitrogen (I will ignore the ocean for now) was root nodules in some species of plants. There are bacteria that live in these nodules which convert N2 (gaseous nitrogen) from the atmosphere into NH3 (ammonium) which the plant can easily use. It’s a symbiotic relationship where the plant supplies a cozy environment for the bacteria as well as carbohydrates from photosynthesis while the bacteria produce ammonia which is quickly converted into amino acids. The reason for the nodule is that the bacterial enzymes which do the N2 ==> NH3 reaction, called “nitrogenases”, are poisoned by oxygen. The nodule provides an environment relatively free of O2 so the enzymes can work efficiently.
On a related tangent, one of the primary ingredients in fertilizer is ammonium nitrate. This is produced through the Haber process where natural gas is used to heat gaseous N2 up to high temperatures in the presence of a catalyst to make various nitrogen compounds. Supposedly around 2% of the world’s total energy supply is used in this single chemical reaction, although I have yet to actually find solid data on this.
Anyways, farmers spray this stuff in their fields to improve yield but a lot of it goes to waste as it runs off into waterways causing toxic algae blooms and eventually into the sea where it causes more problems for ocean life. Back to the story…
For these bacteria and their nitrogenases to work they need an oxygen free environment. So you can’t just take the bacteria and coat a plant with it and hope for it to work.
Only some species of plants have nodules to sustain nitrogen fixation. And unfortunately the plants that humans grow in the largest abundance (maize, rice, wheat) don’t have nodules. People have been trying to engineer root nodules into non-nodulating species for some time without much luck. Turns out it’s quite a complex phenomenon. Ideally you could make a plant produce nodules so farmers don’t have to dump so much fertilizer everywhere, either by some special seed coating or through genetic modification. But so far, no luck. The only thing I have seen recently that could be promising is this company – azotictechnologies.com I kind of know what they are working on but I have yet to see any data showing that it can replace fertilizer.
One possible way to sidestep this nodule problem is to come up with a nitrogenase which is oxygen tolerant. Then you wouldn’t have to deal with engineering or inducing nodulation. You could imagine if you had such a system you could possibly just put the gene straight into the plant so there is no bacteria needed, or maybe make a seed coating containing some bacterial spores carrying this gene, etc. Something way simpler than making root nodules and less polluting and wasteful than fertilizer.
Back in the 90s there was this professor in Germany, Ortwin Meyer, who happened to find a bacteria that ostensibly fixed nitrogen in the presence of oxygen. He and his team were actually looking for bacteria that reduced carbon monoxide and discovered this nitrogenase by accident. In Germany people make charcoal by burying firewood and igniting underground so it undergoes pyrolysis, expelling all sorts of nasty gases and leaving behind charcoal. Meyer et al took some soil samples from atop one of these charcoal “heaps”, for lack of a better word. One of the bacteria he isolated from the soil consumed carbon monoxide but also fixed nitrogen. They called it Streptomyces thermoautotrophicus.
At some point all of the students and postdocs left the lab and no one carried on the project studying the nitrogenase function. I contacted everyone who had ever worked on it and they all claimed to no longer have the species. I don’t believe them, but it’s irrelevant. So it seemed that the species was lost. After getting the runaround from all those folks I decided to try to re-isolate the species myself. This is where I got incredibly lucky. I should have bought a lotto ticket this day. I was talking to one of my German friends, Dirk, about this project trying to determine if I had committed some cultural faux pas which might explain why these scientists were being difficult and cagey. He told me that his dad’s neighbor owns a charcoal “factory” in the region where S. thermoautotrophicus had been isolated. Dirk gave him a call and it turns out this guy owns the exact property where Meyer had isolated that species. So this guy sends me a soil sample from one of his charcoal heaps for me to try to re-isolate this species from.
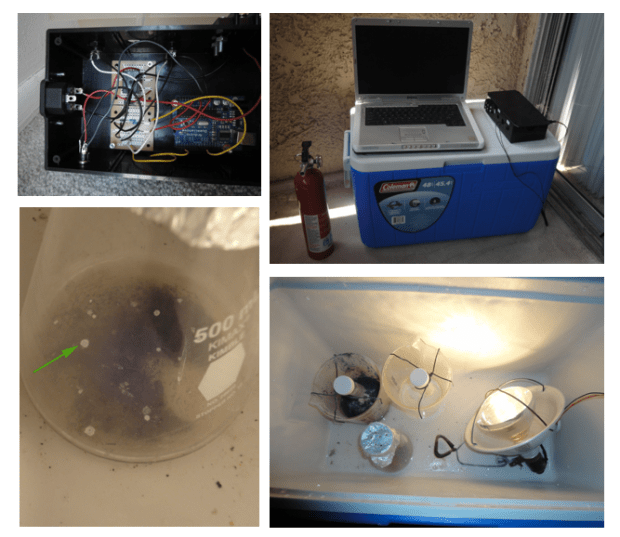
This brings up the question of how do I go about re-isolating the bacteria. From the literature I knew the basic approach was to place the soil sample in a flask, cover it with a little bit of water containing some salts, minerals and other micronutrients, heat it up to 65C and then pump in the gases which this species loves to eat. It consumes either carbon monoxide or a combination of hydrogen and carbon dioxide. Since working with CO in my apartment (this was all done DIY style) was out of the question I went with H2/CO2. My initial design involved an airtight growth chamber with heating element, hydrogen produced from electrolysis of water and CO2 delivered from a canister, through some tubes and valves connected to the chamber. This thing was a complete disaster and I never got it to run for more than a couple of days before some part became too corroded from the high temperature, humidity and corrosive gas.
My second design was centered around a plastic cooler that I picked up from Target. There was a heat lamp inside controlled via a relay and an Arduino which maintained the balmy 65C temperature. The soil sample sat inside a flask with the appropriate mix of vitamins and minerals. To generate the gases I had 2 large plastic cups, 1 for hydrogen and 1 for carbon dioxide. To produce hydrogen I placed aluminum powder in the cup, then poured in 1M NaOH. To produce carbon dioxide I went kindergarten-science-fair-style and mixed baking soda and vinegar. So I would dump the liquids into the powders and then shut the cooler lid quickly to trap the gases in. I would do this twice a day to keep the concentration of the gases up. Eventually I got some bacteria to grow which matched the description of the original species. So I had isolated what appeared to be the original O2-tolerant nitrogen fixing bacteria.
In the meantime, since I had been publishing all this on a public wiki, I got the attention of a couple of other scientists who were interested in helping out. Our collaboration has managed to confirm that this bacteria is indeed fixing nitrogen. We did this by growing it in the presence of isotopic 15-N2, which is a non-radioactive isotope of nitrogen that occurs very rarely in nature. We extracted proteins from the bacteria and ran it though a mass spectrometer. The mass spec showed that the 15N was incorporated into proteins so we know it’s fixing nitrogen. We have also sequenced the genome of this bacterium which turned out more difficult than initially planned, but it’s mostly done. Now the goal is twofold: 1) figure out which genes code for the nitrogenase and 2) determine if the nitrogenase is in fact O2 tolerant. The original research on this species was inconclusive so we have to make sure.
Right now we’re addressing #1 by using RNA-Seq to basically measure which genes are turned on when the bacteria is forced to fix its own nitrogen (be removing ammonia from the growth media). So we grow one sample with ammonia and one without, then measure the level of expression for all the genes (RNA-Seq), compare the 2 samples and see which ones are turned up when ammonia is removed. These genes *should* be the ones involved in the nitrogenase pathway but biology is always messy and complicated so it probably won’t be so conclusive. Eventually we will have to make knockouts, where we remove genes that we think are involved and see if it kills the nitrogenase function to be 100% sure.
As for #2, that is on the back burner for now because it will be very difficult to test without #1.
My ultimate goal is to be able to take a set of genes from Streptomyces thermoautotrophicus and put them in another species, either a plant or an algae or something and have that species fix its own nitrogen too. It would be super useful not only in the agriculture sector but also in industrial algae biofuel operations.
So if you got antsy and skipped to the end, the goal is to engineer biological systems to not need nitrogen-based fertilizer. We’re still a ways away from that goal but making steady progress.
If you have any questions let me know. I’m always happy to talk science. Sorry for the novella.
Cory is a perfect example of the type of professional who is taking advantage of the new amateur tools and models. I suspect this trend will only grow: projects like Cory’s will succeed on Kickstarter or Experiment, biospaces (a new one just opened next door to us in Berkeley) will start producing hugely relevant projects and, hopefully, grant-making foundations will realize there’s big potential in seeding a large number of these low-cost amateur projects with some form of micro-grant, and more post docs like Cory will start their own maker-style projects. That’s my hope, at least.
ADVERTISEMENT










