This project first appeared in Make: Volume 09. Subscribe for the latest projects!
Hold out your palm face up. In the time it takes you to read this sentence, two high-energy subatomic particles will likely have pierced clean through your hand.
We live in a radioactive environment and there’s no escape. At sea level, the flux is roughly one particle per palm size per second, which means San Franciscans sustain an astonishing 20 million hits through their bodies each day. Denver is higher in elevation, so its protective blanket of air overhead is thinner. As a result, Mile Highers get hit at roughly twice that rate.
These particles make their presence felt by ionization — that is, ripping electrons away from their atoms. Since atomic electrons are electrically charged, they interact with all other charged particles (e.g. other electrons, protons, helium nuclei, and so on), and so any of these, when sent screaming through space, becomes a type of ionizing radiation. In addition to charged particles, some photons, such as X-rays and gamma rays, also have enough oomph to whack electrons out of their atomic orbitals and so they, too, make the list.
Much of the radiation around us comes from nuclei in the soil and the air that have too much energy to be stable; they shed their excess by expelling elementary particles. But most of the damaging corpuscles have their origins deep in outer space where pulsars, supernovae, and even stranger cosmic players accelerate elementary particles to fantastic energies and hurl them out into the cosmos. After perhaps 100,000 years of silently streaking between the stars, some of these tiny wanderers end their existence when they strike the nucleus of an atom in the air high over our heads, and explode.
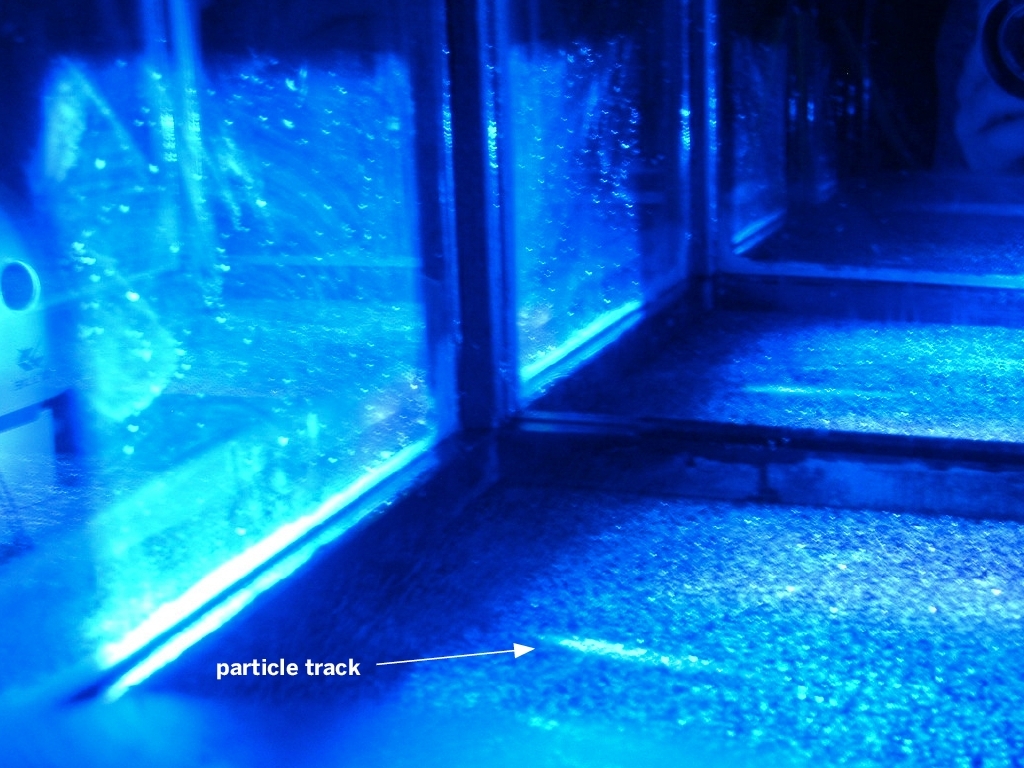
Since energy and mass are two sides of the same coin, some of the enormous kinetic energy these particles often carry instantly converts into wholly new particles, sometimes thousands of them, that shower down toward Earth. Most of these new particles interact with still more atomic nuclei and quickly range out — never reaching the ground.
But one exotic species, the muon, does not feel the nuclear force. As a result, muons do not get absorbed in the air. Most survive to reach Earth’s surface where they fall from the sky in all directions as a steady spray of subatomic machine gun bullets that we call “cosmic rays.”